Last Updated on November 9, 2023 by pm_author_91ksj
6.1 Organizing The Elements Answers Key
As a seasoned blogger with a keen interest in the world of science, I’ve spent years delving into the intricacies of the periodic table. The 6.1 organizing the elements answer key is a topic that’s close to my heart. It’s a fascinating study that unravels the methodical and systematic approach scientists took to organize the myriad of elements we know today.
When I first came across the concept of organizing elements, it struck me as an immense task. Think about it, with over a hundred known elements, how do you start to categorize them in a way that makes sense? The 6.1 organizing the elements answer key provides the answers, shedding light on the ingenious system put in place.
In this article, I’ll share my insights into the 6.1 organizing the elements answer key. We’ll delve into the history, the patterns, and the principles that guide the organization of elements. Whether you’re a science enthusiast or a student looking to ace your next exam, this article will provide a clear, concise, and comprehensive understanding of the topic.

What is Organizing the Elements?
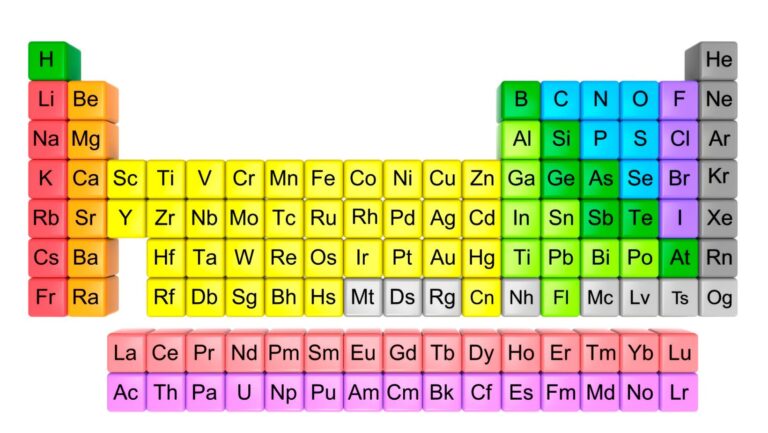
Organizing the elements is the meticulous process of classifying and grouping the elements found in nature according to their properties. This inventive yet analytical endeavor has fascinated scientists for centuries, forcing them to come up with methods to satiate their scientific curiosity.
They’ve worked tirelessly to uncover the underpinning principles and patterns that dictate the behavior and characteristics of these elements. It’s not all random chaos—you’d be amazed at the degree of predictability and regularity when elements are arranged thoughtfully! Every single element has a designated spot in the periodic table, making it easier to understand its behavior and interaction with other elements.
Mendeleev’s Periodic Law was the pioneering breakthrough in organizing elements. When Mendeleev arranged elements in increasing atomic masses, he found that elements with similar properties repeated at regular intervals, a periodicity he subsequently attributed to atomic structure. This insight was groundbreaking, paving the way for the modern Periodic Table we know and study today.
The Modern Periodic Table, structured according to atomic numbers, is a definitive testament to humanity’s collective scientific endeavor. It’s an organized, orderly tableau that neatly categorizes 118 confirmed elements, from the lightest Hydrogen to the superheavy, artificially synthesized Oganesson.
Hydrogen, Helium, Lithium, and all the way down to Oganesson, every element has its rightful place, clearly defined and distinguished by its group and period.
With every periodic table, students, researchers, and scholars unlock a world of elemental relationships and patterns—an unrivaled tool that keeps revealing the secrets of the universe, one element at a time.
Why is Organizing the Elements Important?
Organization, in any context, is key to making sense of the vast amount of information available. Applying this concept to the elements might have been one of the most pivotal milestones in the progress of scientific knowledge. Here we’ll delve into the specific reasons why organizing elements into the Periodic Table was, and continues to be, an imperative task in the field of chemistry.
Facilitates Easy Access
One of the many benefits to having a well-structured table like the Periodic Table is that it simplifies access to information. With over a hundred confirmed elements, the Periodic Table acts as an index, allowing scientists, students, and enthusiasts to quickly locate and study elements.
Possessing such a directory reduces the time and effort spent scavenging through scattered data. You’ve got altogether 118 confirmed elements conveniently displayed on one table. Imagine having to rummage through individual records for each of those elements. Not exactly a scenario you’d want to get caught up in, right?
Let’s add a layer to this and look at it from the perspective of a student studying for exams. The Periodic Table streamlines study sessions by presenting all pertinent information upfront. Atom counts, valance electrons, you name it, it’s there. This organization essentially works as a perfect cheat sheet for your chemistry-related quandaries.
Provides Clarity and Structure
The Periodic Table’s structure isn’t merely about ease of access. It’s far more significant than that. It’s about relationships. It’s about patterns. It’s about predicting behaviors of elements, both known and yet undiscovered. Yes, the Periodic Table can do all that!
The arrangement of elements into groups and periods is a clear indication of shared characteristics and atomic behaviors. All elements in a group exhibit similar physical and chemical properties. Similarly, patterns in atomic behavior are clear as day when moving across a period.
This organization of elements allows for the easy prediction of an element’s properties and behaviors based on its position in the table. This means even undiscovered elements can be assigned anticipated characteristics based on where they’d end up in the table, upon discovery.